A next generation of stem cell applications
Our Mission
American Regenerative Medicine Company aims to provide clinicians with a comprehensive set of off-the-shelf regenerative medicine products for significant unmet clinical needs.
Creating tomorrow's personalized medicine
American Regenerative Medicine Corporation provides clinicians with a comprehensive set of off-the-shelf regenerative medicine products for significant unmet clinical needs, based on the successful results of Dr. Minjie Gu's adult stem cell therapy clinical trials in China.
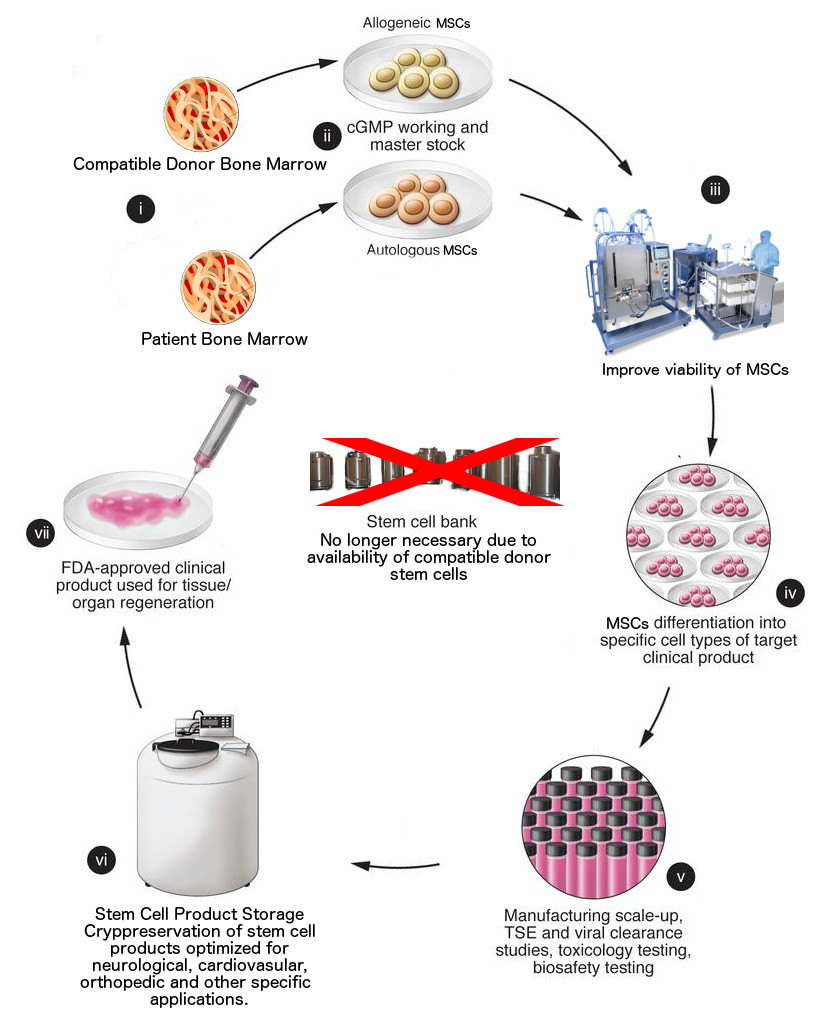
Stem cell technology promises to provide treatments for many formerly intractable conditions. The Company's goal is to take advantage of the power of this emerging area of biotechnology to provide treatments for acute diseases, injuries, chronic diseases, and anti-aging; with an initial focus on developing kits to treat five disease areas: neurological, orthopedic, cardiovascular, cancer and several systemic diseases.
The Company's products will enable production of therapeutic amounts of several types of highly purified regenerative stem cells from patients or donors, based on Dr. Gu's proprietary cell-based core technologies.
The Company expects to conduct several clinical trials and obtain CFDA (China) product approvals during the next 12 months and completion of additional trials and obtaining of approvals from the FDA (U.S.A.) and other countries the following year.
American Regenerative Medicine Corporation is being organized in the State of New York as a pharmaceutical company to provide clinicians with a comprehensive set of personalized and off-the-shelf regenerative medicine products (kits), based on the successful results of Dr. Gu's adult stem cell therapy clinical trials in China.
The Company's market is 21st century human health engineering through clinical applications of stem cell technologies that promises to provide personalized treatments for over 90% of difficult diseases. Our goal is to be an important part of the market for personalized regenerative medicine, which is growing exponentially and creating demand for new medicines.
The Company's proprietary cell-based core technologies include methods for producing highly purified, immunoselected Mesenchymal Precursor Cells (MPCs), culture-expanded Mesenchymal Stem Cells (MSCs), Dental Pulp Stem Cells (DPSCs), Adipose Stem Cells (ASCs), expanded Hematopoietic Stem Cells (HSCs) and combination dendritic (DCs)/cytokine-induced killer (CIKs) cells. The Company's protein generation technologies are based on factors derived from Dr. Gu's proprietary cell-based core technologies.
The Company plans to build and operate a sales department, an R&D department, a quality control department, a clinical trials department for regenerative medicine treatments based on these products, as well as a manufacturing facility for full-scale production of these products.
American Regenerative Medicine Corporation is located in New York City. Dr Minjie Gu is President and Chief Scientist.
Copyright © 2016 - All Rights Reserved - American Regenerative Medicine Corporation
Template by OS Templates